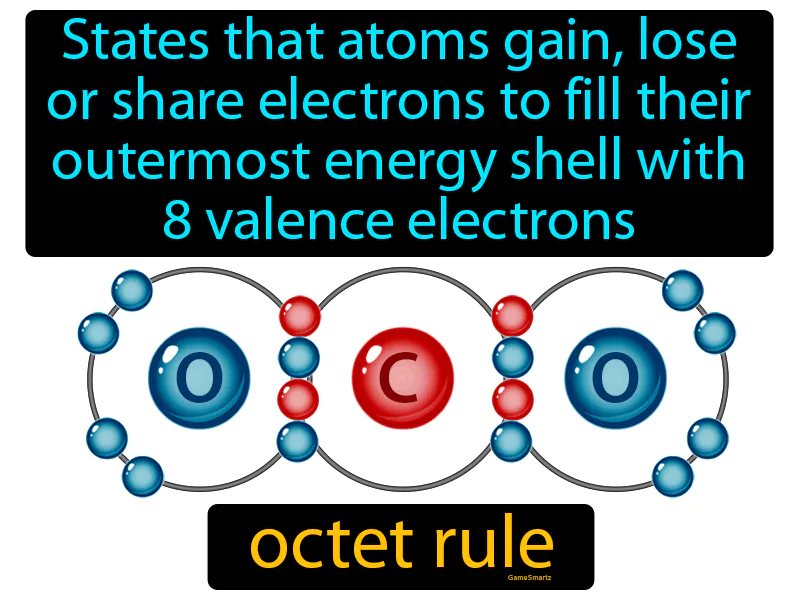
Octet Rule
Octet Rule:
Imagine trying to assemble a jigsaw puzzle, where the picture only looks complete when all the pieces are perfectly placed. In a similar way, atoms strive to achieve a stable configuration by having eight electrons in their outermost shell, just like completing the puzzle with all pieces. Just as a puzzle is incomplete without all its pieces fitting together, an atom feels "incomplete" without a full set of valence electrons, so it gains, loses, or shares electrons to achieve that full "puzzle" of eight, resulting in stability.
Practice Version
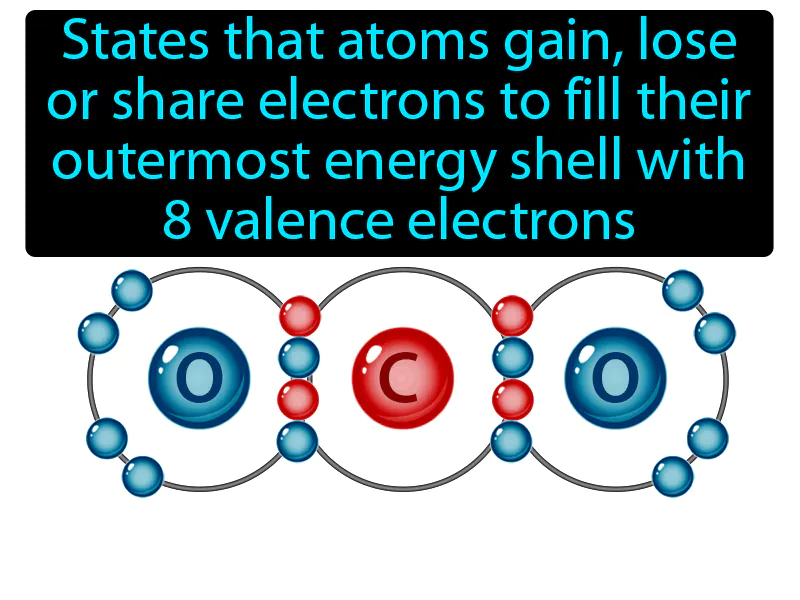
Octet Rule: States that atoms gain, lose or share electrons to fill their outermost energy shell with 8 valence electrons. Octet rule. The octet rule is a guideline that atoms are most stable when they have eight electrons in their outer shell.