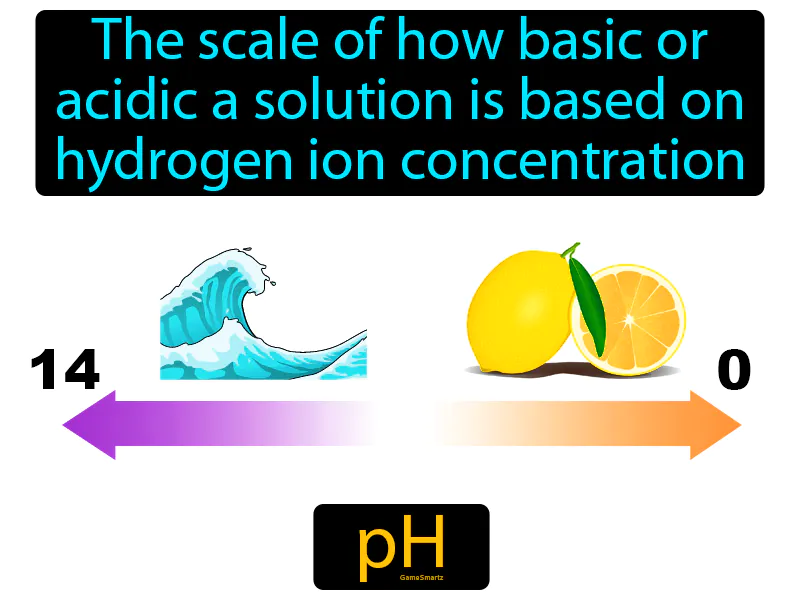
PH
PH Meaning:

Example:
Imagine you're trying to find just the right amount of seasoning to add to a soup to make it taste perfect. Just like how the amount of salt or spice determines whether your soup is bland, perfectly seasoned, or too salty, the pH scale measures how acidic or basic a solution is based on its hydrogen ion concentration. In this analogy, the seasoning is like the hydrogen ions: too few make the solution basic (like a bland soup), just the right amount makes it neutral, and too many make it acidic (like an overly salty soup).
Practice Version

PH: The scale of how basic or acidic a solution is based on hydrogen ion concentration. pH. pH measures how acidic or basic a solution is, with lower values being more acidic and higher values more basic.