Hydrogen Bond
Hydrogen Bond Meaning:
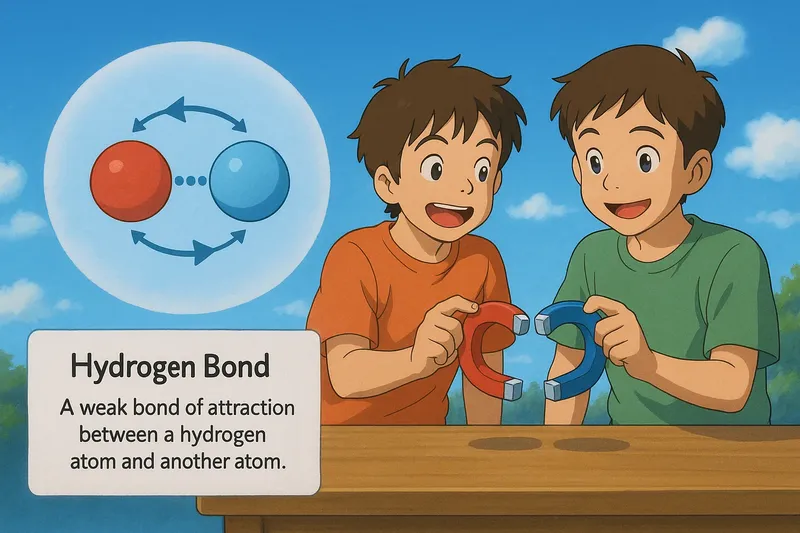
Example:
Imagine trying to keep two magnets close together on a table without them snapping together or drifting apart. This is similar to how a hydrogen bond works, where a hydrogen atom is attracted to another atom, like oxygen or nitrogen, but the bond is not strong enough to completely pull them together. Just as the magnetic pull keeps the magnets in close proximity without them fully sticking, a hydrogen bond keeps molecules close but not fully bonded, maintaining a delicate balance of attraction.
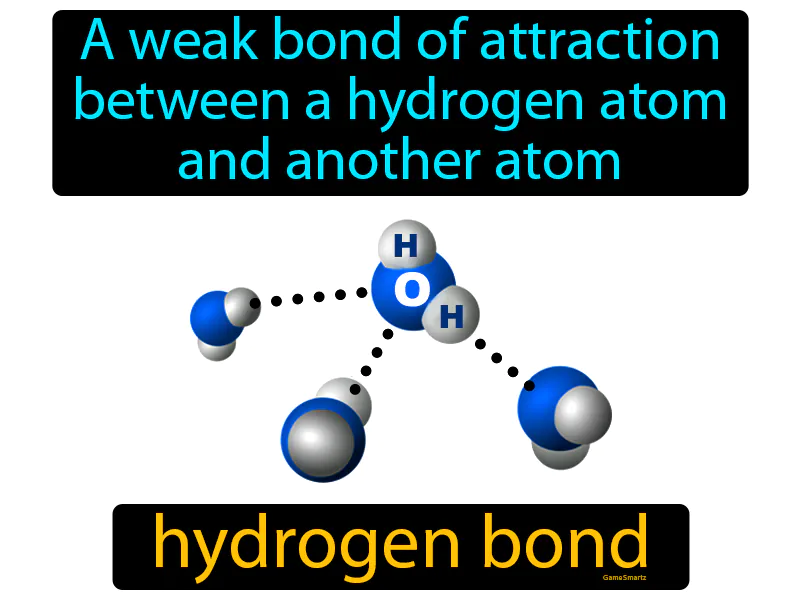
Practice Version
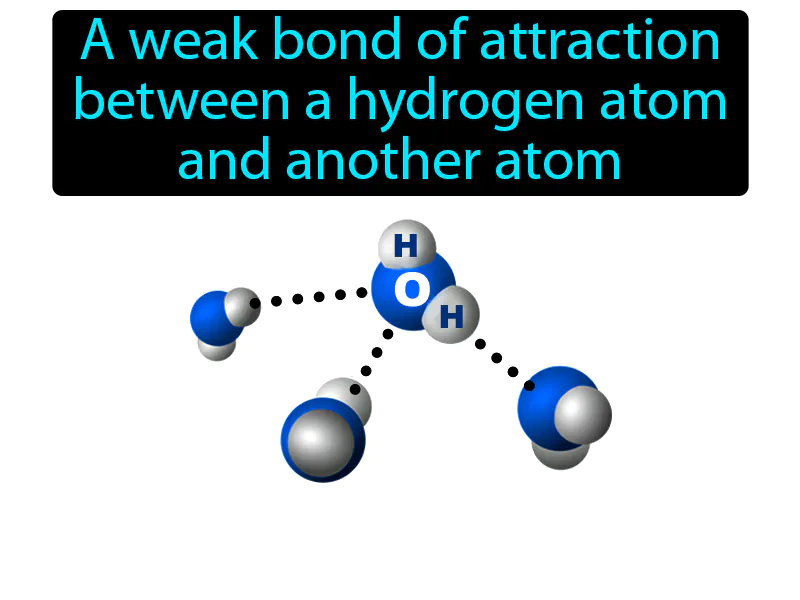
Hydrogen Bond: A weak bond of attraction between a hydrogen atom and another atom. Hydrogen bond. In simple terms, a hydrogen bond is a weak connection where a hydrogen atom on one molecule is attracted to an electronegative atom on another molecule.