Equilibrium Constant
Equilibrium Constant:
Imagine you’re trying to keep a room at the perfect temperature with both a heater and an air conditioner. Balancing the temperature is like maintaining the equilibrium in a chemical reaction, where the heater represents the reactants and the air conditioner represents the products. Just as the equilibrium constant indicates the balance between reactants and products in a chemical reaction, the thermostat setting reflects the balance needed to keep the room comfortable by managing the output of both appliances.
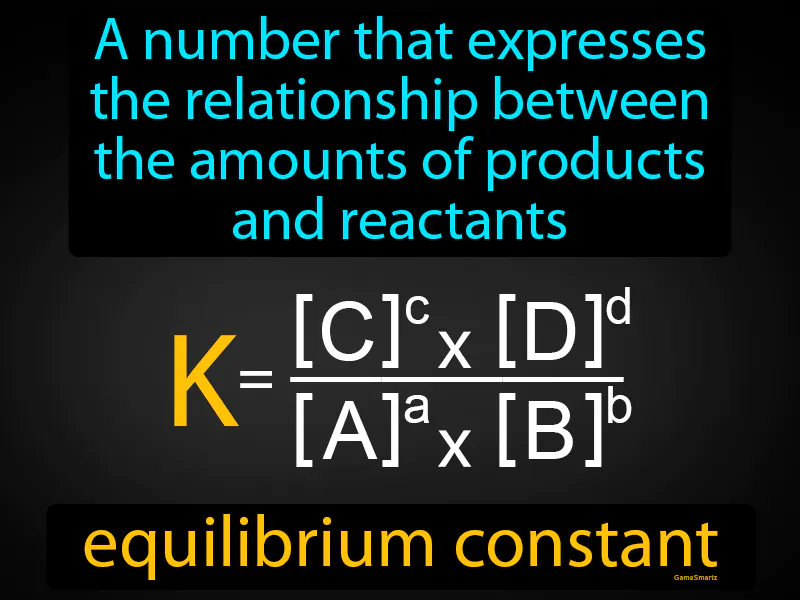
Practice Version
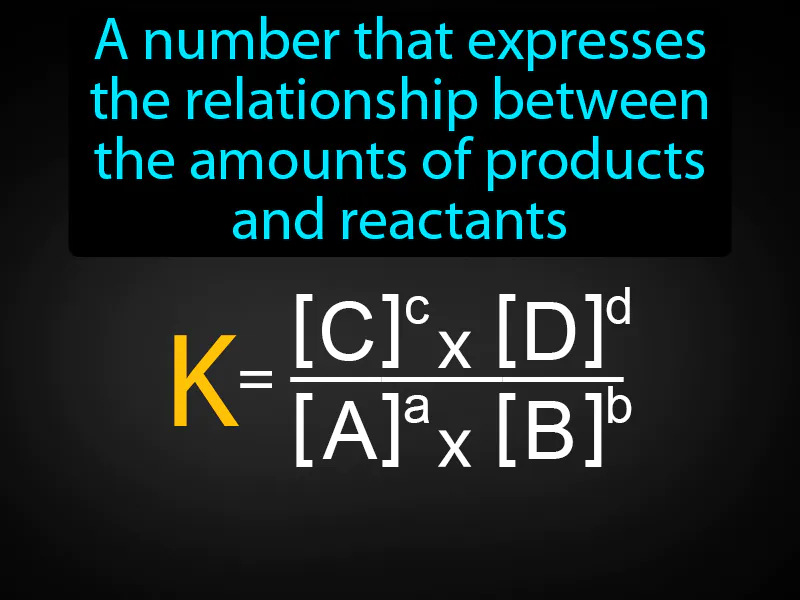
Equilibrium Constant: A number that expresses the relationship between the amounts of products and reactants. Equilibrium constant. The equilibrium constant is a number that shows the ratio of concentrations of products to reactants at equilibrium in a chemical reaction.