Geometric Isomer
Geometric Isomer:
Imagine you're trying to assemble a bookshelf, but the shelves can be positioned in two different ways, leading to either a stable or a wobbly setup. This situation is similar to geometric isomers, where compounds have the same atoms and bonds but differ in how groups are oriented around a double bond, much like how shelves can be positioned differently. Just as the stability of the bookshelf depends on the orientation of its shelves, the chemical properties of geometric isomers depend on the orientation of groups around the double bond, highlighting the critical role of spatial arrangement.
Practice Version
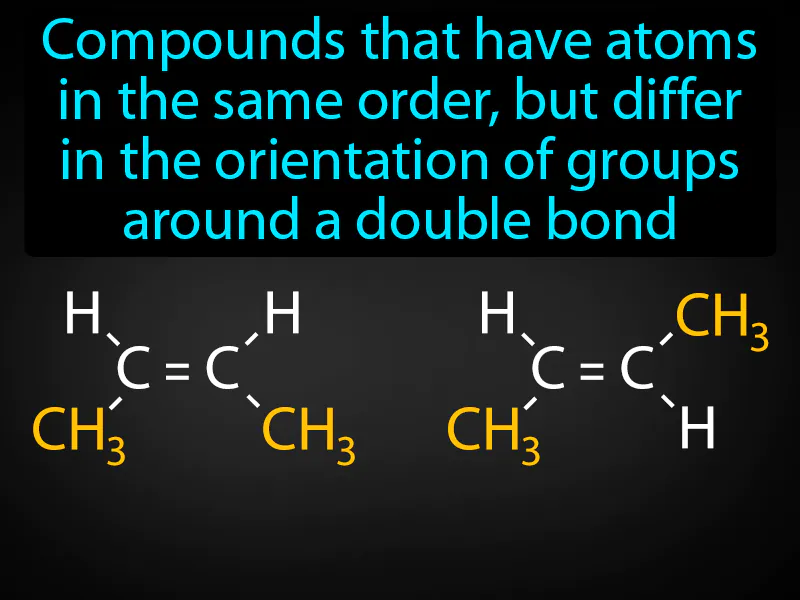
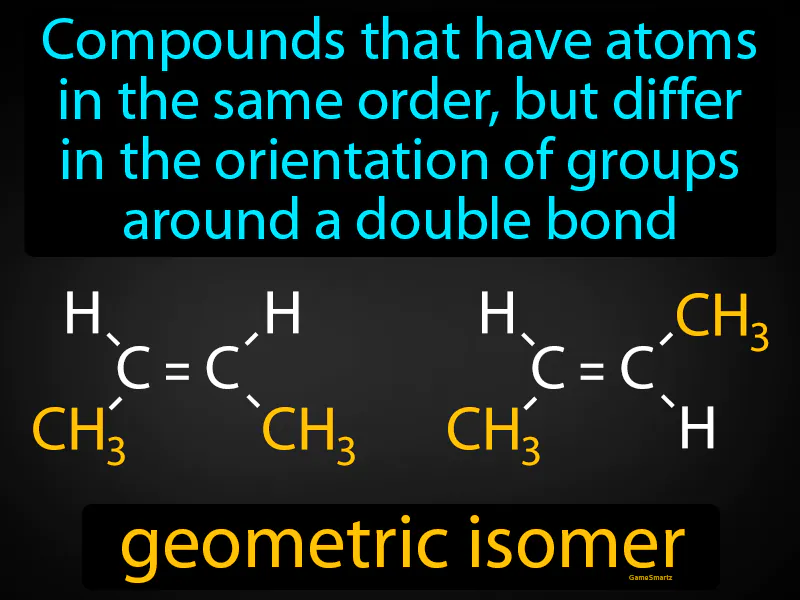
Geometric Isomer: Compounds that have atoms in the same order, but differ in the orientation of groups around a double bond. Geometric isomers are molecules that have the same structure but different spatial arrangements of atoms due to the rigidity of double bonds.