Unsaturated Hydrocarbon
Unsaturated Hydrocarbon Meaning:

Example:
Imagine you're trying to find a parking spot in a busy grocery store lot, and the only available spaces are the ones that require you to make tight maneuvers. This situation is similar to the structure of an unsaturated hydrocarbon, where carbon atoms are connected by double or triple bonds, creating less flexibility and more tension in the molecule. Just as those tight parking spots require precise navigation because of limited space, the double or triple bonds in unsaturated hydrocarbons create specific angles and rigidity, making the molecule less adaptable compared to single-bonded, saturated hydrocarbons.

Practice Version
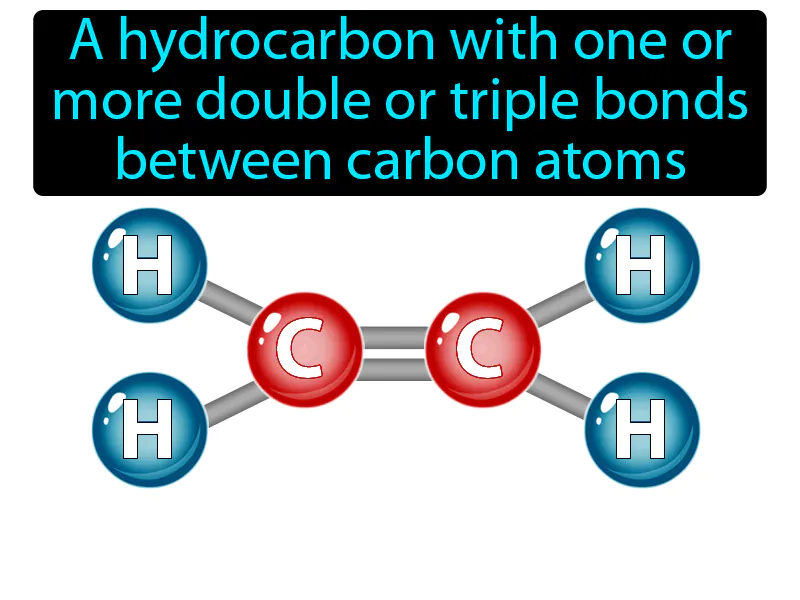
Unsaturated Hydrocarbon: A hydrocarbon with one or more double or triple bonds between carbon atoms. Unsaturated hydrocarbon. An unsaturated hydrocarbon is a molecule made of carbon and hydrogen containing double or triple bonds.