Benzene
Benzene:
Imagine trying to organize a group of friends for a dinner where everyone wants to sit in a circle, maintaining a balanced and harmonious conversation. Just like finding the perfect arrangement where each friend is equally engaged and connected, benzene is structured in a way that its carbon atoms form a perfect ring, maintaining stability and balance in its chemical bonds. In this analogy, the friends represent the carbon atoms, the circular seating arrangement symbolizes the cyclic structure of benzene, and the harmonious conversation reflects the stability provided by the aromatic nature of benzene’s electron cloud.

Practice Version
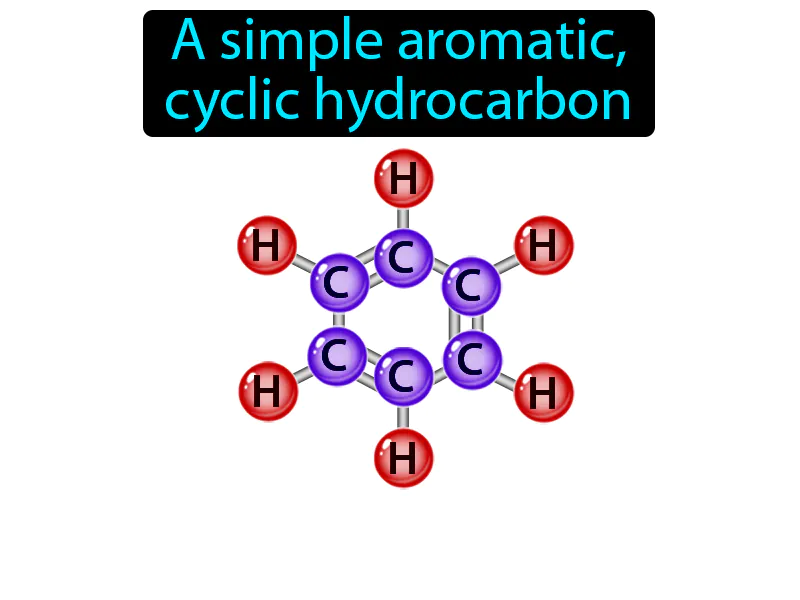
Benzene: A simple aromatic, cyclic hydrocarbon. Benzene. Benzene is a ring-shaped molecule made of six carbon atoms and six hydrogen atoms, commonly used as a starting material in making plastics, resins, and other chemicals.