Specific Heat
Specific Heat Meaning:
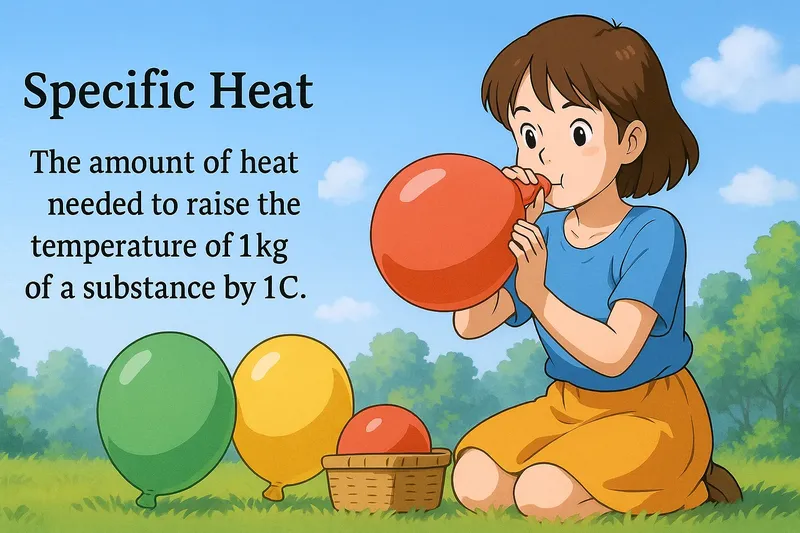
Example:
Imagine you’re trying to fill a balloon with air, and you notice that different balloons require different amounts of air to reach the same size. This is similar to the concept of specific heat, where different substances need different amounts of heat to increase their temperature by 1°C. Just as each balloon has a unique capacity determining how much air it can hold before reaching a certain size, each substance has a specific heat capacity dictating how much heat it requires to increase its temperature by a specific amount.

Practice Version

Specific Heat: The amount of heat needed to raise the temperature of 1kg of a substance by 1C. Specific heat. Specific heat is a measure of how much heat energy is required to change the temperature of a substance.