Freezing Point
Freezing Point Meaning:
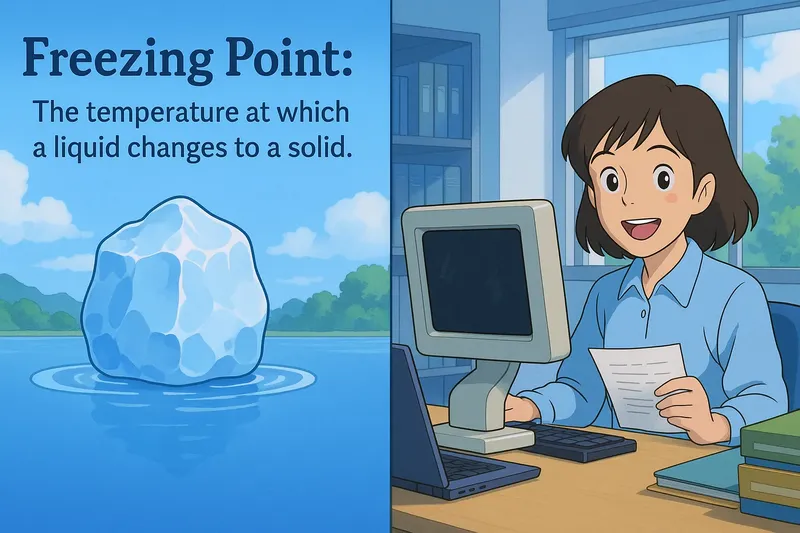
Example:
Imagine you're juggling multiple tasks at work, and suddenly, everything comes to a standstill when your computer crashes. This situation is similar to the freezing point in chemistry, where a liquid transitions to a solid when the temperature drops to a certain level. Just as your productivity halts when your computer stops functioning, the movement of liquid molecules slows and eventually stops, locking them into a fixed position as they form a solid at the freezing point.

Practice Version

Freezing Point: The temperature at which a liquid changes to a solid. Freezing point. The freezing point is the temperature at which a liquid becomes a solid.