Valence Electron
Valence Electron Meaning:
Example:
Imagine you're at a party, and you're the person standing near the door, ready to meet new people and make connections. Just like you're positioned to easily interact and form new friendships, valence electrons are positioned in the outermost energy level of an atom, ready to form chemical bonds. In this analogy, you are the valence electron, the party is the atom, and the new people you meet are the atoms or molecules that bond with you, showing how valence electrons are crucial for forming bonds, much like you are crucial for forming social connections at the party.
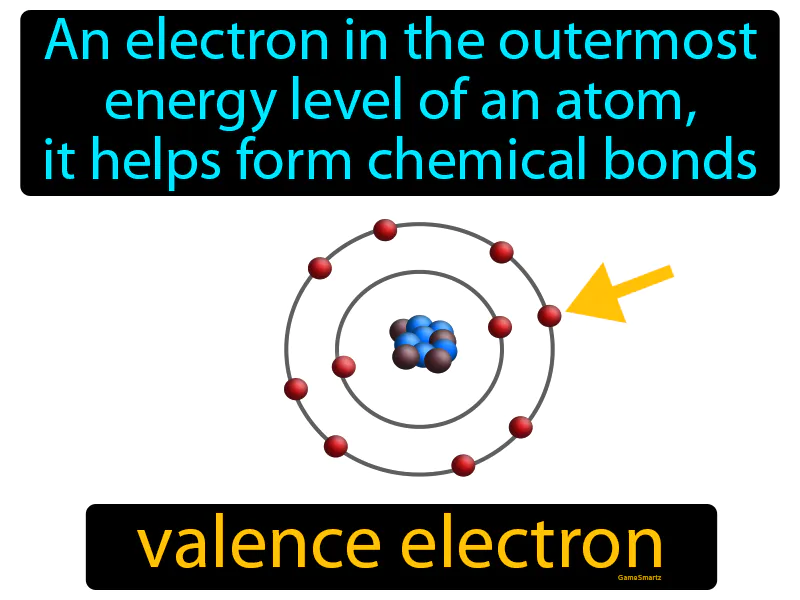
Practice Version
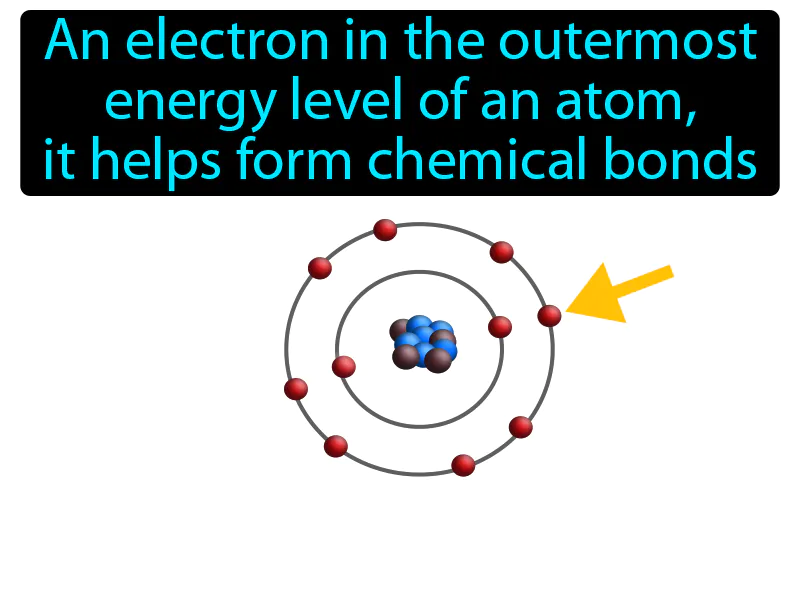
Valence Electron: An electron in the outermost energy level of an atom, it helps form chemical bonds. Valence electron. A valence electron is an electron that can participate in forming chemical bonds with other atoms.