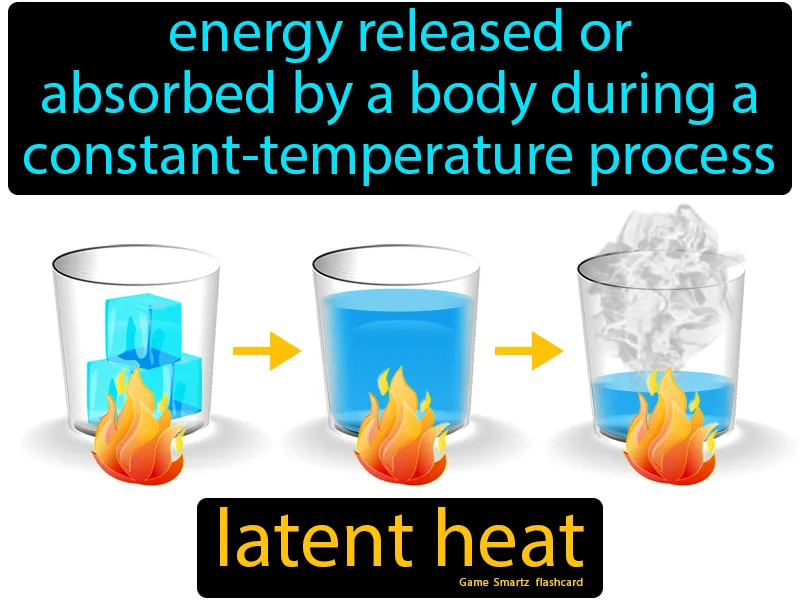
Latent Heat
Latent Heat:
Imagine trying to melt a block of ice with your hands on a cold day, noticing that it takes a while even though you're continuously applying warmth. This is similar to the concept of latent heat, where energy is absorbed or released during a phase change without a change in temperature. Just as your hands must constantly supply heat to the ice until it fully melts, latent heat involves energy being absorbed during the phase change from solid to liquid, even though the temperature of the ice remains constant until the process is complete.
Practice Version
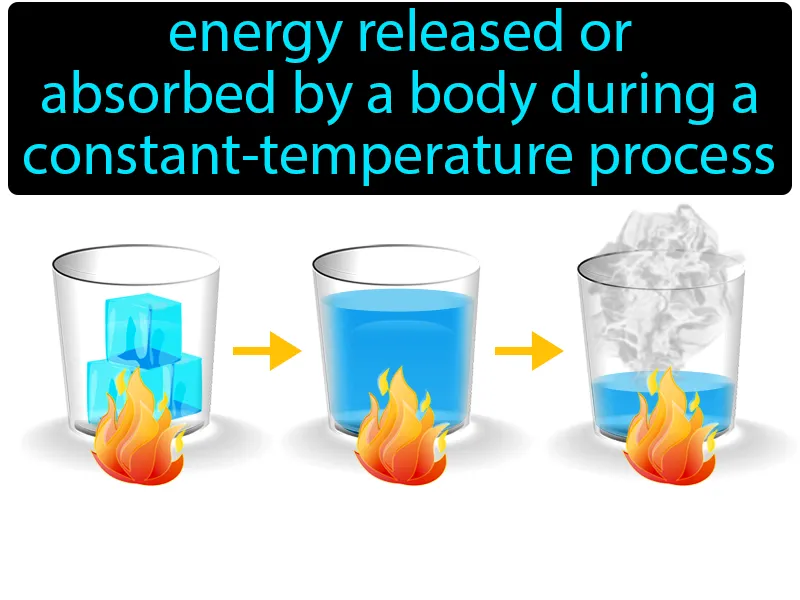
Latent Heat: Energy released or absorbed by a body during a constant-temperature process. Latent heat. It is the energy required to change the state of a substance without changing its temperature.