Nuclear Equation
Nuclear Equation:
Imagine you're trying to balance a checkbook, ensuring that every dollar you earn and spend is accurately accounted for. Balancing a nuclear equation is similar because it requires ensuring that the total atomic numbers and mass numbers on both sides of the equation are equal, just like you need to balance credits and debits in your checkbook. In this analogy, the atomic number is like the individual transactions—each must be accounted for to maintain balance—while the mass number is akin to the total amount of money, ensuring that what you started with equals what you end up with, just as in a balanced checkbook.
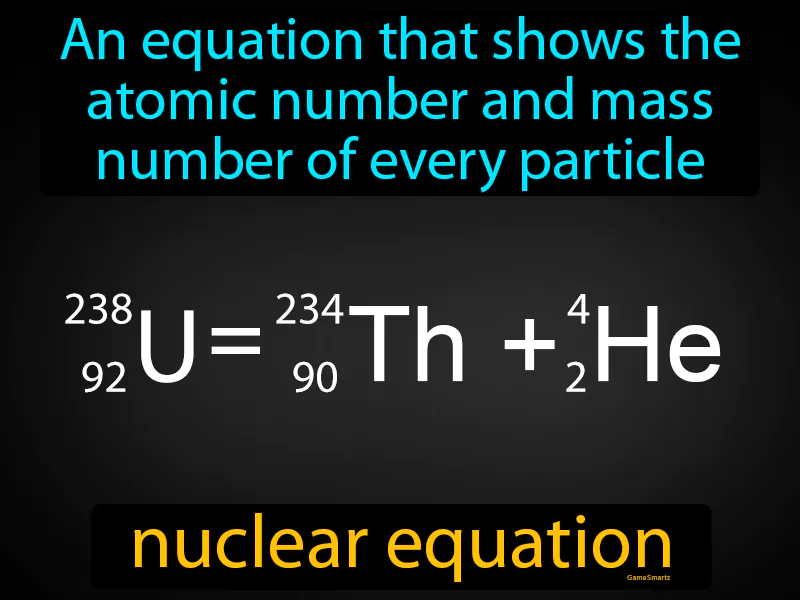
Practice Version
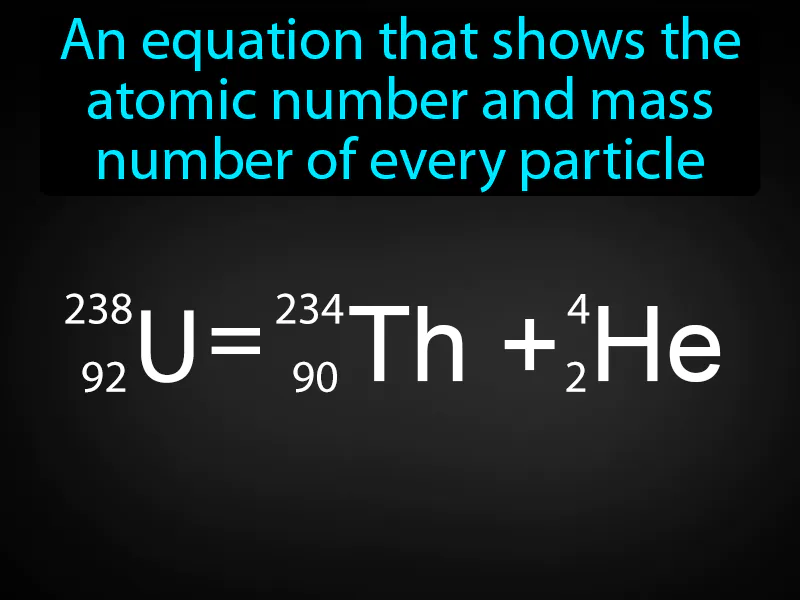
Nuclear Equation: An equation that shows the atomic number and mass number of every particle. Nuclear equation. A nuclear equation is a representation of a nuclear reaction showing the atomic numbers and mass numbers of the particles involved.