Henrys Law
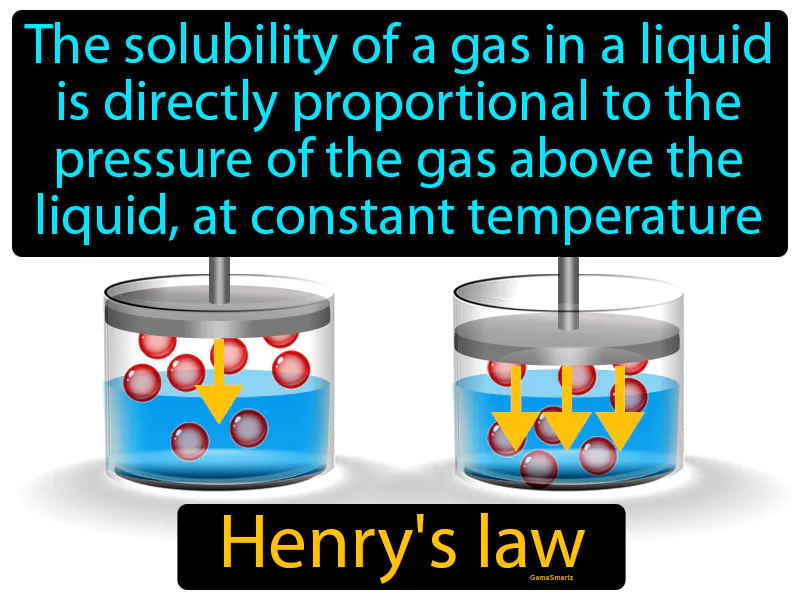
Henrys Law:
Imagine trying to keep a lid on a fizzy drink to prevent it from going flat. Just like how the pressure from the lid keeps the fizz in your drink, Henry's Law explains that the more pressure you apply to a gas above a liquid, the more of that gas will dissolve into the liquid. The lid is like the pressure in Henry's Law, where increased pressure keeps more gas dissolved, just as the higher pressure from the sealed lid keeps your drink fizzy by preventing the carbon dioxide from escaping.
Practice Version
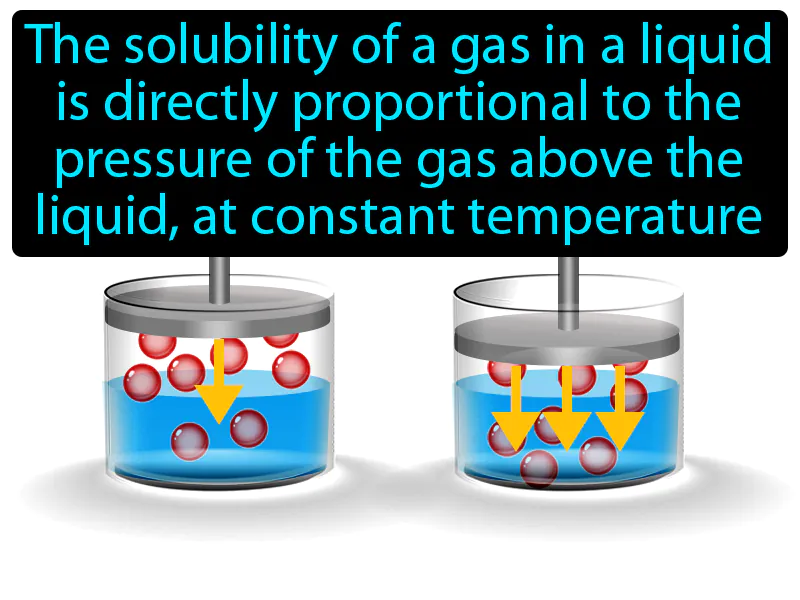
Henrys Law: The solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid, at constant temperature. Henry's law. Henry's law means that if you increase the pressure of a gas above a liquid, more of that gas will dissolve into the liquid.